강추이종목
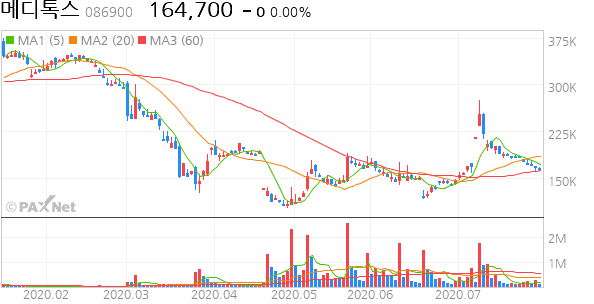
[메디톡스] 메디톡스 코로나 백신임상 1상 성공 !!!! 3개월 안에 임상3상

최강네트워크
조회980
현 가격대에선 단기, 중기,장기 매수해 볼 가격대
아직 안알려진 뉴스
BUSINESS NEWS
JULY 29, 2020 / 10:19 PM / UPDATED 9 HOURS AGO
Vaxine expects to start Phase II trials for potential COVID-19 vaccine in weeks
Divya Chowdhury
(Reuters) - Australian biotechnology company Vaxine Pty Ltd expects to start Phase II trials of its potential COVID-19 vaccine in the next few weeks after “positive” results from the first stage human study, chairman Nikolai Petrovsky said on Wednesday.
FILE PHOTO: A woman holds a small bottle labeled with a "Vaccine COVID-19" sticker and a medical syringe in this illustration taken April 10, 2020. REUTERS/Dado Ruvic/File Photo
Final stage trials could start within the next three months, he told the Reuters Global Markets Forum chat room in an exclusive interview.
“It’s extremely safe and well-tolerated. We already have that data from the clinical trial. We’ve not had any bad reactions to the vaccine. That’s very positive,” he said.
The company is working with South Korea’s MedyTox (086900.KQ), according to the World Health Organization’s list of candidate vaccines. Human trials on 40 people started this month.
No vaccine has yet been approved for COVID-19, the disease caused by the new coronavirus that has killed more than 659,000 people and unleashed economic havoc worldwide.
Vaxine’s shot is one of more than two dozen at the human clinical trial stage as the race to tame the pandemic heats up.
Petrovsky said two years is a realistic but ambitious time frame to get a COVID-19 vaccine to market, underscoring the challenge facing the global drugs industry to help control the virus.
“We may have vaccines that look effective after one year, but to then manufacture billions of doses and get them to people, I think everybody expects two years,” he said.
“If you look at measles and polio, where there was a desperate urgency, it still took 4-5 years to develop an effective vaccine.”
In terms of pricing, Petrovsky said COVID-19 vaccines could potentially cost between $20 to $200 per dose, depending on the technology’s manufacturing cost. He said more effective vaccines, or those with fewer or no side effects may sell at a higher price.
Pfizer’s deal with the U.S. government to sell 100 million doses for $2 billion “indicates that the price for a very large volume of vaccine will be around $20, but potentially on the private market, it may be $40-$50 by the time you have mark-ups by chemists and you get to retail,” Petrovsky said.
((This interview was conducted in the Reuters Global Markets Forum, a chat room hosted on the Refinitiv Messenger platform. Sign up here to join GMF: refini.tv/2LbSKPl))
Reporting by Divya Chowdhury; Editing by Josephine Mason and Kirsten Donovan
Our Standards:The Thomson Reuters Trust Principles.
REUTERS NEWS NOW
Subscribe to our daily curated newsletter to receive the latest exclusive Reuters coverage delivered to your inbox.
Enter email address
MORE FROM REUTERS
AppsNewslettersAdvertise with UsAdvertising GuidelinesCookiesTerms of UsePrivacy
All quotes delayed a minimum of 15 minutes. See here for a complete list of exchanges and delays.
? 2020 Reuters. All Rights Reserved.
×
Better questions. Better answers.
GET THE APP
JULY 29, 2020 / 10:19 PM / UPDATED 9 HOURS AGO
Vaxine expects to start Phase II trials for potential COVID-19 vaccine in weeks
Divya Chowdhury
(Reuters) - Australian biotechnology company Vaxine Pty Ltd expects to start Phase II trials of its potential COVID-19 vaccine in the next few weeks after “positive” results from the first stage human study, chairman Nikolai Petrovsky said on Wednesday.
FILE PHOTO: A woman holds a small bottle labeled with a "Vaccine COVID-19" sticker and a medical syringe in this illustration taken April 10, 2020. REUTERS/Dado Ruvic/File Photo
Final stage trials could start within the next three months, he told the Reuters Global Markets Forum chat room in an exclusive interview.
“It’s extremely safe and well-tolerated. We already have that data from the clinical trial. We’ve not had any bad reactions to the vaccine. That’s very positive,” he said.
The company is working with South Korea’s MedyTox (086900.KQ), according to the World Health Organization’s list of candidate vaccines. Human trials on 40 people started this month.
No vaccine has yet been approved for COVID-19, the disease caused by the new coronavirus that has killed more than 659,000 people and unleashed economic havoc worldwide.
Vaxine’s shot is one of more than two dozen at the human clinical trial stage as the race to tame the pandemic heats up.
Petrovsky said two years is a realistic but ambitious time frame to get a COVID-19 vaccine to market, underscoring the challenge facing the global drugs industry to help control the virus.
“We may have vaccines that look effective after one year, but to then manufacture billions of doses and get them to people, I think everybody expects two years,” he said.
“If you look at measles and polio, where there was a desperate urgency, it still took 4-5 years to develop an effective vaccine.”
In terms of pricing, Petrovsky said COVID-19 vaccines could potentially cost between $20 to $200 per dose, depending on the technology’s manufacturing cost. He said more effective vaccines, or those with fewer or no side effects may sell at a higher price.
Pfizer’s deal with the U.S. government to sell 100 million doses for $2 billion “indicates that the price for a very large volume of vaccine will be around $20, but potentially on the private market, it may be $40-$50 by the time you have mark-ups by chemists and you get to retail,” Petrovsky said.
((This interview was conducted in the Reuters Global Markets Forum, a chat room hosted on the Refinitiv Messenger platform. Sign up here to join GMF: refini.tv/2LbSKPl))
Reporting by Divya Chowdhury; Editing by Josephine Mason and Kirsten Donovan
Our Standards:The Thomson Reuters Trust Principles.
REUTERS NEWS NOW
Subscribe to our daily curated newsletter to receive the latest exclusive Reuters coverage delivered to your inbox.
Enter email address
MORE FROM REUTERS
AppsNewslettersAdvertise with UsAdvertising GuidelinesCookiesTerms of UsePrivacy
All quotes delayed a minimum of 15 minutes. See here for a complete list of exchanges and delays.
? 2020 Reuters. All Rights Reserved.
×
Better questions. Better answers.
GET THE APP
무료 전문가 방송
1/3
최근 방문 게시판
실시간 베스트글
베스트 댓글
-
남아있던 물량도 3.40 위로 대부분 청산되어 이제 잠을 청할까 합니다 조금만 자고 일어나야합니다 이시간 모두 행복한 시간이길 바랍니다
ELW토론야간 마무리 ~
-
 선옵토론실게시판 조두순
선옵토론실게시판 조두순 -
 선옵토론실어느듯 52돈 팔찌에
선옵토론실어느듯 52돈 팔찌에 -
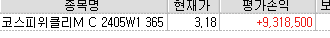 ELW토론야간 마무리 ~
ELW토론야간 마무리 ~ -
이야 미국넘 선물을 끌어 올릴 정도로 기도의 효험이 대단 하내 기도가 안통한줄 알았는데 통하내
선옵토론실할렐루야~~~



0/1000 byte